More Information
Submitted: April 18, 2023 | Approved: May 03, 2023 | Published: May 04, 2023
How to cite this article: Ruvalcaba-González AP, Escalera-López Fde J, Macias-Ortega BI, Araujo-Conejo A, Clinical characteristics of patients with respiratory disease and probable COVID-19 at the General Hospital Zacatecas Mexico. Arch Clin Exp Orthop. 2023; 7: 007-014.
DOI: 10.29328/journal.aceo.1001014
Copyright Licence: © 2023 Ruvalcaba-González AP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Comorbidities; COVID-19; Diabetes mellitus (DM); Systemic arterial hypertension (SAH); Obesity (OB); SARS-CoV-2
Abbreviation: SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease 2019; PCR: Polymerase Chain Reaction; DM2: Type 2 Diabetes Mellitus; SAH: Systemic Arterial Hypertension; OB: Obesity; OR: Odds Ratio; RR: Relative Risk; ICU: Intensive Care Unit; nm: nanometers; UV: Ultraviolet Rays; MV: Mechanical Ventilation; FRE: Risk Factor by age; SISVER: Respiratory Disease Surveillance System; RLB: Binary Logistic Regression; BMI: Body Mass Index; RD: Respiratory Disease; UV: Ultraviolet Rays; SD: Standard Deviation; Ho: Hypothesis of nullity; df: degrees of freedom; IMSS: Instituto Mexicano del Seguro Social; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; SSA: Servicios de Salubridad y Asistencia; SPSS: Statistical Package for the Social Sciences; CDC: Center for Disease Control
Clinical characteristics of patients with respiratory disease and probable COVID-19 at the General Hospital Zacatecas Mexico
Ruvalcaba-González AP1, Escalera-López Fde J1, Macias-Ortega BI1 and Araujo-Conejo A2*
1Department of Teaching and Research of the General Hospital Zacatecas “Luz González Cosío” of the Health Services of Zacatecas, Mexico
2Research Department of the Health Services of Zacatecas, Mexico
*Address for Correspondence: Araujo-Conejo A, Research Department of the Health Services of Zacatecas, Mexico, Email: [email protected]
Introduction: The spread of SARS-CoV-2 cases grew exponentially. In Mexico, it focused mainly on containing the disease and adopting activities and actions to mitigate it. Hospital reconversion was a fundamental strategy in the management of care for patients with COVID-19.
Objective: To know the clinical characteristics of patients admitted with respiratory disease and probable COVID-19 in the Zacatecas General Hospital “Luz González Cosío” México.
Material and methods: Descriptive, cross-sectional, and analytical study, at the General Hospital, from March 2019 to September 2021; using data from the Respiratory Disease Surveillance System. Data from patients admitted with a diagnosis of some pathology of respiratory disease and probable COVID-19 were analyzed.
Results: We included 2,678 diagnosed with respiratory disease and a mean age; of 47.6 ± 21.6 gender distribution was almost equal; women 1,344 (51.0%). positive result to COVID-19 by PCR; 1,654 negatives; 900 and 124 without result. 193 (7.0%) required mechanical ventilation. The presence of comorbidities was evaluated; type 2 diabetes mellitus, systemic arterial hypertension, obesity, alone and together. Also the association of the age factor, as well as the lethality index; was 531 (19.6%).
Discussion: What has been published in other studies about comorbidities and their influence on the severity of COVID-19 is confirmed, disagreeing on the case fatality rate; 20.7% against what was reported; 17.6% in other countries for COVID-19 hospitalized. An age variable was used as a risk factor with a cut-off point > 45 years; (FRE), obtaining; RR 3.42 (95% CI 2.79 to 4.19) and an odds ratio of 4.015 in binary logistic regression analysis. Reported male mortality (OR = 1.45; 95% CI: 1.41–1.51) according to our OR results; 1,45.
Conclusion: The present study shows how certain chronic diseases influenced respiratory disease to present a serious state, regardless of the positive or negative result of COVID-19.
In December 2019, the World Health Organization (WHO) was alerted for some cases of pneumonia reported in Wuhan China, of unknown cause at the time. SARS-CoV-2 was identified as the cause of COVID-19. Currently, with much more information and research about it, it is known that it is a positive polarity RNA virus, whose size ranges between 60 nm - 220 nanometers (nm) [1], with a lipid envelope, very sensitive; heat, antiseptics, ultraviolet rays (UV) and highly contagious. Spreading rapidly around the world, WHO declared the outbreak a public health emergency in late January 2020 [2].
Infections by this virus reached about 168 million cases in 191 countries in May 2021 [3,4]. This pandemic seriously affected Latin America after Asia and Europe, the first case reported was on February 26, 2020, in Brazil, and subsequently, the first death occurred in Argentina in March of the same year [4]. The Country of Mexico reported its first case on February 25, 2020 and the first death occurred on March 18 of the same year. Mexico with an approximate population of 128,649,565 million inhabitants, by the month of May 2.5 million people had already been infected and 224,000 deaths from COVID-19 represented an approximate mortality ratio of 10% [5,6].
Due to this situation, measures were implemented in Mexico to mitigate the spread of the pandemic, as there was a declaration of national emergency, consisting of implementing some restrictions in the different sectors; public, private, and social, in addition to including a voluntary quarantine limiting non-essential activities. On the other hand, within this emergency plan, a reconversion of hospital services was proposed, consisting of the organization of care units in the national territory in order to respond to the COVID-19 pandemic in Mexico [7]. Therefore, the units that were not converted continued with normal care, only some activities of the hospital decreased.
Despite all efforts to control the pandemic, there was an increase in the number of cases, especially severe cases. There were reports indicating that about 50% of the affected global population had at least one chronic disease, increasing the risk of severe COVID-19, in addition, there are studies where it is mentioned, that almost a third of patients admitted to the ICU had a history of a greater number of comorbidities. On the other hand, being > 65 years old influenced the progression and prognosis of severe COVID-19 [8,9].
Body mass index (BMI) is strongly correlated with the behavior of COVID-19, however, some studies on comorbidities only focused on identifying age and chronic diseases such as systemic arterial hypertension (SAH), cardiovascular disease, and cancer as risks for severe COVID-19 [10,11]. Subsequently, studies were published suggesting that patients with obesity would be at increased risk for developing severe COVID-19 [12]. There are studies that present obesity as a risk factor for hospitalization and ICU admission, as well as complications with severe and potentially fatal consequences of COVID-19 [13]. On the other hand, an underlying chronic disease could be aggravated and have future consequences.
The degree of severity, as well as mortality from COVID-19, associated with particular medical conditions, determine considerable differences in the national and global epidemiological setting. Since a population with risk factors, but with little or no access to health services, becomes more vulnerable to transition to severe COVID-19, it has a greater impact on COVID-19 pandemic mortality [14,15]. In low- and middle-income countries, socioeconomic status is sometimes associated with obesity, whereas, in high-income countries, the opposite is often true. There is strong evidence that people with chronic degenerative diseases, such as diabetes, should be considered a risk factor for rapid progression and poor prognosis of COVID-19 [16,17].
The U.S. Center for Disease Control (CDC), in 2020 reported that of 1,482 adult patients hospitalized with COVID-19, 180 (12.1%) had some comorbidity; among the most common were; HAS 49.7%, Obesity; 48.3%, Diabetes; 34.6% and cardiovascular disease; 27.8%. The prevalence of obesity in Mexico affects about 30% of the adult population, which is strongly associated with cardiovascular disease and type 2 diabetes (DM2) [18]. It should also be noted that Mexico is among the top 10 countries with patients suffering from DM2, affecting about 9.2% of the adult population [19]. Therefore and due to the above, the present study was carried out.
A descriptive, observational, cross-sectional, and analytical study was conducted at the Zacatecas General Hospital “Luz González Cosío” of the Health Services of Zacatecas, Mexico, in the period from March 2019 to September 2021. Data obtained from the Respiratory Disease Surveillance System (SISVER) were used. The hospital unit corresponds to a second-level hospital, with 140 beds, which did not participate in the conversion program to a COVID hospital, so it remained offering its care services to the population. He only reduced some of his activities due to the contingency, but maintained emergencies and hospitalization.
The objective of this study was to identify the clinical characteristics of patients admitted with a diagnosis of RD and who, due to the pandemic as a protocol during their hospitalization, were tested for SARS-CoV-2 by PCR, in order to isolate the positive. Because of this, two groups were randomly generated; positive and negative, this being our basis for conducting the study. The variables to be identified were; health status during hospitalization, clinical characteristics and associated comorbidities, severity, and mortality, with the only difference of being positive or negative for COVID-19. Bivariate and multivariate statistical analysis, Pearson’s chi-square, Spearman’s correlation, and binary logistic regression, were performed with IBM® SPSS Statics 24 for Windows. The study was approved by the HGZ Research Ethics Committee.
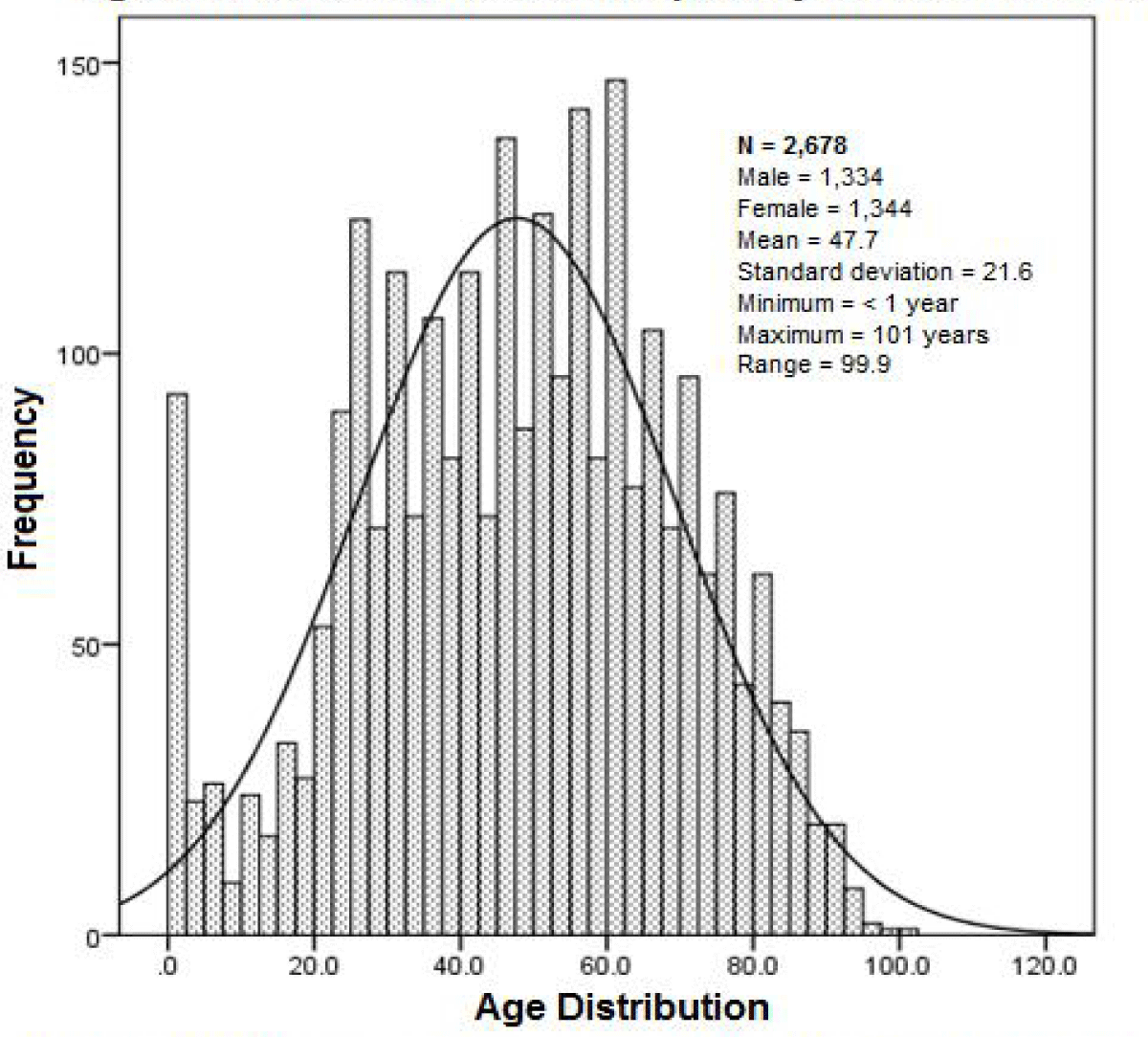
A total of 2,698 patients were identified who were admitted with a diagnosis of RD, during the period; March 2019 to September 2021, 20 of the total were eliminated, due to not knowing their outcome due to their transfer to another unit, leaving an N = 2,678. Most of these patients were tested for SARS-CoV-2, finding; 1,654 (61.8%) positive, 900 (33.6%) negative and no result 124 (4.6%). Its general distribution by age; arithmetic mean of 47.7 years, with a standard deviation (SD) ± 21.6 and with respect to sex; women 1,344 (50.2%) and men 1,334 with mean age and SD; 46.8 ± 21.1 and 48.4 ± 22.1 respectively (Figure 1).
Figure 1: Age of patients admitted for respiratory disease in the HGZ. The histogram shows their reference with respect to their normal distribution, in the age frequencies in general, as well as their statistics.
Patients were distributed by age ranges, based on frequency distribution amplitude (Range = 99.9) resulting in five class intervals for age; Infant (< 2 years), pediatric (≥ 2 to < 18), young adult (≥ 18 to < 40), mature adult (≥ 40 to < 60) and older (≥ 60 and over). With this variable we sought to obtain more information when evaluating its relationship with the variables; gender and death, in a contingency table for proof of independence.
The analysis found a high mortality rate in the older adult range; 31.0% and 40.5% in females and males respectively; Pearson’s chi-square: 88.68 p < 0.000 and Spearman’s correlation: 0.285 p < 0.000 In addition this index was determined for each interval. The test accepts the existence of a mortality dependence on age, regardless of gender (Table 1).
| Table 1: Frequency Distribution by Gender, Age Classification and Mortality of Patients in the Zacatecas General Hospital of the S.S.Z. | |||||||
| Gender Classification | Death | Mortality Rate | Total | ||||
| No | Yes | % | Sig. p < 0.05 | ||||
| Female | Infant | 40 | 5 | 11.1 | 45 | ||
| Pediatric | 67 | 7 | 9.45 | 74 | |||
| Young Adult | 468 | 32 | 6.40 | 500 | |||
| Mature Adult | 364 | 103 | 22-05 | 467 | |||
| Older | 178 | 80 | 31.0* | 258 | |||
| Total Partial | 1117 | 227 | 16.8 | 1344 | p < 0.000 | ||
| Male | Infant | 42 | 6 | 12.5 | 48 | ||
| Pediatric | 52 | 6 | 10.3 | 58 | |||
| Young Adult | 423 | 45 | 9.0 | 468 | |||
| Mature Adult | 311 | 109 | 25.9 | 420 | |||
| Older | 202 | 138 | 40.5* | 340 | |||
| Total Partial | 1030 | 304 | 22.7 | 1334 | p < 0.000 | ||
| Total | Infant | 82 | 11 | 11.8 | 93 | ||
| Pediatric | 119 | 13 | 9.84 | 132 | |||
| Young adult | 891 | 77 | 7.95 | 968 | |||
| Mature adult | 675 | 212 | 23.9 | 887 | |||
| Adulto Mayor | 380 | 218 | 36.4* | 598 | |||
| Grand Total | 2147 | 531 | 19.8 | 2678 | p < 0.000 | ||
| Here it is observed how the mortality rate increase, starting from the class interval; in mature adults, reaching its máximum in older adults, 40.5% with a general contingency value; of 0.270 and p < 0.000. | |||||||
Due to the mortality observed in patients aged > 45 years, it was proposed to generate a risk variable with this cut-off point, use it as an age risk factor (REFRE), and identify its association with the other variables. The PCR result gives us an idea of the SARS-CoV-2 viral load, regardless of the clinical signs of COVID-19, however, we evaluate this PCR result and mortality, with and without FRE, in order to identify its influence. Finding a frequency of patients with FRE of; 1,485 (55.4%) and 1,193 (44.6%) without FRE, in contrast to the PCR result for SARS-CoV-2, considered positive; 1,654 (61.7%), negative; 900 (33.6%) and no result; 124 (4.6%). Analyzed in a contingency table, obtaining a case fatality rate of; 29.8% for patients with positive FRE and PCR and a rate of; 6.1% without factor FRE, but with positive PCR. In general, the influence of the FRE variable for mortality is observed, to a greater extent than being positive for SARS-CoV-2 with PCR. Concluding that when FRE does not influence, mortality is not independent of the PCR test, Pearson’s Chi-square: p = 0.011 (Table 2).
| Table 2: Evaluation of FRE with the Result of PCR for SARS-CoV-2 and Mortality in Patients of the General Hospital Zacatecas. | |||||||
| FRE factor | SARS-CoV-2 Test | Death | Mortality Rate % |
Total | Sig- p < 0.05 | ||
| No | Yes | ||||||
| Without FRE |
PCR | Positive | 592 | 39 | 6.1 | 631 | |
| Negative | 439 | 54 | 10-9 | 493 | |||
| Without Result | 61 | 8 | 11.5 | 69 | |||
| Total Partial | 1,092 | 101 | 8.4 | 1,193 | 0.011 | ||
| With FRE | PCR | Positive | 718 | 305 | 29.8 | 1,023 | |
| Negative | 299 | 108 | 26.5 | 407 | |||
| Without Result | 38 | 17 | 30.9 | 55 | |||
| Total Partial | 1,055 | 430 | 28.9 | 1,485 | 0.443 | ||
| Total | PCR | Positive | 1,310 | 344 | 20.7* | 1,654 | |
| Negative | 738 | 162 | 18.0* | 900 | |||
| Without Result | 99 | 25 | 20.1* | 124 | |||
| Grand Total | 2,147 | 531 | 19.8 | 2,678 | 0.237 | ||
| Risk factor; FRE with cut-off point > 45 years and PCR result for SARS-CoV-2, related to mortality, dependence with statistical significance is observed only when the FRE factor is not present; Pearson's chi-square; 9.060 and p = 0.011 | |||||||
With regard to comorbidities, we identify; diabetes mellitus (DM2), systemic arterial hypertension (SAH), and obesity (OB) as well as, their combinations: (DM2 + SAH), (OB + SAH), (OB + DM2), and (MD2 + SAH+OB). We looked for its relationship with mortality, and it was found that there is dependence between the variables of comorbidities with mortality, evaluated with Pearson’s Chi-square test; Value; 92,030 p < 0.000 the highest case fatality rate corresponded to the variable; (DM2+SAH+OB) with 46 deaths in 138 cases (33.3%) (Table 3).
| Table 3: Comorbidities and their Frequency Rate for Mortality. | ||||
| Comorbidity | Death | Mortality Rate % |
Total | |
| No | Yes | |||
| Without Comorbidity | 677 | 83 | 10.9 | 760 |
| DM2 | 113 | 43 | 27.5 | 156 |
| SAH | 211 | 85 | 28.5 | 296 |
| OB | 293 | 66 | 18.3 | 359 |
| MD2+SAH+OB | 92 | 46 | 33.3* | 138 |
| DM2+OB | 37 | 11 | 22.9 | 48 |
| SAH+OBE | 128 | 43 | 25.1 | 171 |
| DM2+SAH | 175 | 69 | 28.2 | 244 |
| Other Pathologies | 421 | 85 | 16.7 | 506 |
| Grand Total | 2147 | 531 | 19.8 | 2678 |
| This table shows that the highest mortality rate is when a patient suffers from the three comorbidities DM2, SAH, and OB, being the highest rate. Pearson's Chi-square; Value 92.03 and p < 0.000 | ||||
In the evaluation of the case fatality rate, for patients who used mechanical ventilation (MV) and died, it was identified that according to gender; 56.1% and 61.0% corresponded to women and men respectively and a case fatality rate of 58.7% for all patients with MV. With statistical significance and contingency coefficient of 0.260 and p < 0.000 and a value; of 193.2 for testing; Pearson’s chi-square p < 0.000 description in the contingency table for independence test (Table 4).
| Table 4: Use of Mechanical Ventilation by Gender and Mortality Rate. | |||||||
| Gender | Death | Total | Rate | Sig. | |||
| No | Yes | % | p < 0.05 | ||||
| Female | Ventilation | No | 1072 | 177 | 1249 | 14.2 | |
| Yes | 39 | 50 | 89 | 56.2* | 0.000 | ||
| Total Partial | 1111 | 227 | 1338 | 17.0 | |||
| Male | Ventilation | No | 990 | 242 | 1232 | 19.6 | |
| Yes | 39 | 61 | 100 | 61.0* | 0.000 | ||
| Total Partial | 1029 | 303 | 1332 | 22.7 | |||
| Total | Ventilation | No | 2062 | 419 | 2481 | 16.9 | |
| SI | 78 | 111 | 189 | 58.7 | |||
| Grand Total | 2140 | 530 | 2670 | 19.9 | 0.000 | ||
| Here we observe the mortality rate with respect to the need to use mechanical ventilation, and its distribution by gender, finding a significant association for mortality, in both genders, being higher in men. Pearson's chi-square p < 0.000. | |||||||
Then, patients requiring MV were; 189 (7.0%) of a total; 2,670 individuals, of whom 101 (58.7%) died, hence the relative risk (RR) of MV use and death was determined; RR = 3.48 (95% CI 3.0 – 4.03) with an OR of; 7.0 (95% CI 5.15 – 9.53). On the other hand, the behavior of mortality in MV and the presence of FRE was identified, determining that; 133 (70.3%) had FRE, of whom died; 80 (60.1%) contrasted with 56 (29.7%) who did not have FRE; where they died; 31 (55.3%). With these data we calculate the RR, to die having; VM and FRE finding; RR = 1.09 (95% CI 0.83 -1.43) that is to say, an absolute risk reduction (RR) of; -4.79% (95% - 20.5% CI to 10.66%).
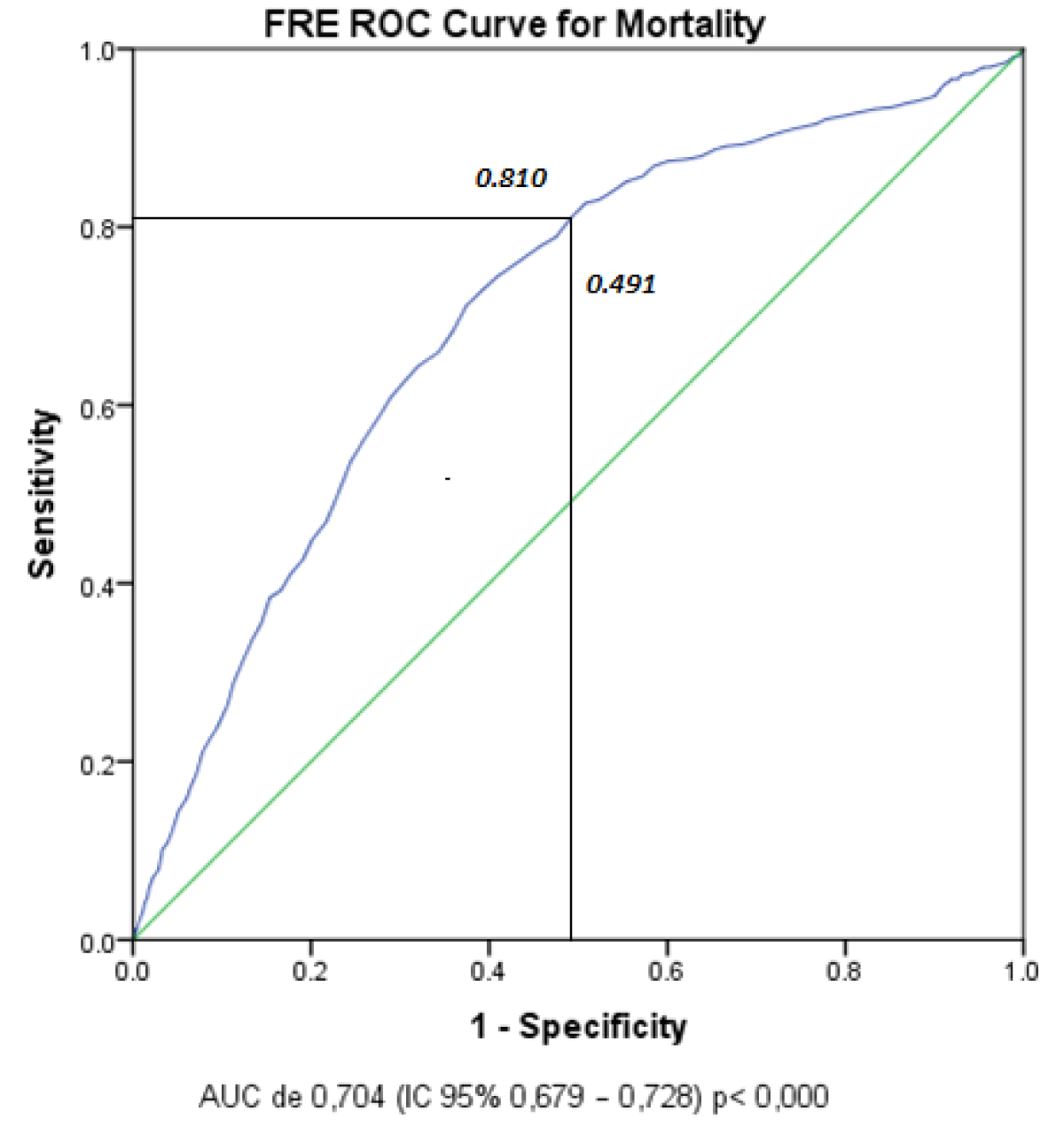
Through a multivariate analysis in the binary logistic regression (RLB) model, we sought to evaluate the factors associated with mortality. Using the independent variables associated with mortality, with statistically significant value (p < 0.05) in the test; Likelihood ratio and Pearson’s Chi-square. The final model included the following independent variables: PCR test, mechanical ventilation, comorbidities, age > 45 years (FRE), gender, and as a dependent variable mortality. The results showed that the positive PCR test obtained the lowest Odds Ratio (OR); 1,150 as well as the highest OR; 6,587 for VM (Table 5). It was because of these results, that we evaluated the FRE cut-off point and made a ROC curve to identify the area under the curve (AUC), finding: an AUC of 0.704 (95% CI 0.679 – 0.728) p < 0.000 These values found mean; Sensitivity of 81% and specificity of 59.9% (Figure 2).
| Table 5: Multivariate Analysis of Binary Regression. | ||||||
| B | Standard Error |
Wald | df | Sig. | Odds Ratio | |
| PCR Categorical Variable | 2.246 | 2 | 0.325 | |||
| Negative Test | 0.264 | 0.246 | 1.149 | 1 | 0.284 | 1.302 |
| Positive Test | 0.140 | 0.115 | 1.471 | 1 | 0.225 | 1.150 |
| Ventilation | 1.885 | 0.167 | 128.148 | 1 | 0.000 | 6.587 |
| Comorbities | 0.416 | 0.139 | 8.907 | 1 | 0.003 | 1.516 |
| FRE | 1.390 | 0.128 | 118.047 | 1 | 0.000 | 4.015 |
| Gender | 0.372 | 0.105 | 12.558 | 1 | 0.000 | 1.451 |
| Constant | -3.089 | 0.165 | 350.851 | 1 | 0.000 | 0.046 |
| In this multivariate analysis, the variables with the greatest influence on the result are presented; alive or dead, where we observe that the categorical variable; PCR for SARS-CoV-2, has no statistical significance, despite having an OR greater than one. However, ventilation and FRE had a higher OR as well as its significance. | ||||||
Figure 2: FRE ROC curve for Mortality. For the evaluation of the FRE cut-off point we perform a ROC curve to identify the area under the curve, which can be observed at the junction of coordinates, for a cut-off point of > 45.
It is pertinent to mention that the present study was carried out in a second-level hospital, which was not transformed for the care of COVID-19 patients, so it continued with its functions at its level, but yes, restricting the influx of patients due to the contingency. Among the patients requiring care were mainly those suffering from acute respiratory infections. Due to this and the pandemic, the demand for service increased, and the lack of a triage program, for COVID-19 patients, admissions occurred according to the urgency. Subsequently, PCR tests were performed to identify SARS-CoV-2 infection, and thus distribute them to an area suitable for them. This gave us the opportunity to evaluate patients with RD, classified as negative or positive for COVID-19. The database of the Respiratory Disease Surveillance System: SISVER, was fundamental for the present study since it gave us the possibility to identify; clinical characteristics and associated factors in patients with RD and who tested positive for COVID-19, adding this variable in the evaluation of mortality. Since, randomly two groups were formed, with the possibility of comparison.
For the analysis, we started by evaluating the distribution of the PCR variable; positive, negative, and without results for COVID-19, through a statistical Chi-square test of goodness of fit, using for its contrast the gender variable, as it was the one that was distributed normally; 50.1% and 49.9% females and 49.9% respectively, to substantiate that the distribution of the categorical variable PCR is presented proportionally in both gender groups. The result of the test; Pearson’s Chi-square Value: 1.046 with 2 degrees of freedom (df) with p = 0.593, represented the non-rejection of the nullity hypothesis: which states that the proportion of the categorical values of the PCR variable is equal in the two categories of the gender variable. The rejection of, the Ho, would only occur with an observed value of Chi-square with an alpha of 0.05 and 2 df, greater than expected of; 61.7 so that not being older, Ho is accepted.
The results of our study confirm that the prevalence of comorbidities increases the risk of dying in hospitalized COVID-19 patients, as they worsen the patient’s clinical conditions [19]. There are results showing that the prevalence of mortality in hospitalized patients with COVID-19 was 17.62% (95% CI: 14.26–21.57%), in 42 studies and 423,117 patients [20]. This contrasts with our results where a case fatality rate of 20.7% was found for COVID-19 patients positive by PCR, and in those negative to the test, but with RD, a rate of; 18.0%, meanwhile, in those who had no result; 20.1%, who could have been patients with RD alone or COVID-19 (Table 2).
Likewise, it is mentioned that advanced age has been shown to be a higher risk of dying due to the coronavirus since an Odds Ratio has been reported in grouped studies, of 2.61 (95% CI 1.75 - 3.47), [19] however we cannot compare it with what was found in our study since we do not use an elderly variable, but we made a cut of > 45 years, based on the distribution in the frequency histogram for age. The cut-off point; Age > 45, we use as a variable; FRE, obtaining; RR 3.42 (95% CI 2.79 to 4.19) and an Odds Ratio of 4.015 in multivariate analysis as a component of a binary logistic regression, using the statistical package for Windows ®IBM SPSS 24. On the other hand, in that same meta-analysis, a significant association between COVID-19 mortality and male gender was reported (OR = 1.45; CI 95% 1.41–1.51) data that, if it agrees with our results because in the analysis we calculate an Odds Ratio of 1.45.
In patients with DM2 infected with COVID-19, the inflammatory process due to infection complicates the insulin response and generates increased endothelial damage. What it can trigger; hyperreactivity in the airways and increase risk of respiratory failure and cardio-pulmonary collapse, as well as possible death [21]. The relationship between; COVID-19, DM, and OB, can be represented; by an inflammatory response that in many cases becomes severe, complicating the picture in these patients. In addition, these patients often suffer from comorbidities, which further worsen clinical outcomes [21]. The first reports of patients with COVID-19 showed that diabetes increased the risk of requiring intensive care and at the same time MV. There are reports that 22.2% of patients in an ICU had diabetes, so they conclude, that it should be considered as a risk factor for the rapid progression and poor prognosis of COVID-19 [16]. In the present study, we did not identify the frequency of patients in ICU, however, the data of the need to use MV in patients with DM, if identified, found a frequency of 9.6%, which was the highest in contrast to OBE and SAH; 8.3% and 6.7% respectively.
With regard to the frequency of comorbidities, regardless of the challenge posed by the COVID-19 pandemic to public health, the presence of these in patients with COVID-19 meant an increased risk of worsening and dying. For the present study, comorbidities were taken into account: DM2, SAH, OB, and other pathologies, among which were; Leukemia, Cancer, Cirrhosis, Rheumatoid Arthritis, Asthma, Hypothyroidism, Chronic Renal Failure, Malnutrition, and Heart Disease, etc. They accounted for; 760 (28.3%) without comorbidities, obtaining a mortality frequency for these patients; 83 (10.9%) and from here they were identified; 55 (66.2%) positive for SARS-CoV-2 with PCR. Table 3 shows the distribution of comorbidities with their receptive mortality frequencies. Among the weaknesses of our study, we find what usually happens with “Retrospective” studies that, when analyzing the data, we find the absence of information necessary to validate some of our results of interest such as, for example; the classification of obesity was only determined; patient with or without obesity; BMI ≥ 30 K/m2 without the possibility of classifying it and identifying its influence on the severity of the condition. On the other hand, it should be remembered that the situation as the patients presented themselves in the study, was particular as commented at the beginning of this discussion.
The present study shows how certain chronic diseases, typical of our population, influenced the RD to present a serious state, regardless of the positive or negative result of COVID-19, since a study that proposed to identify the clinical characteristics of RD, became the description, of the behavior of the disease and its possible COVID-19 infection.
A very high mortality rate could be observed in patients considered only with RD, due to their negative PCR result for COVID-19, since 18.0% to 20.7% in those positive for COVID-19 there is very little difference, and yes, we count those who did not have a result; 20.1% that almost equals the positives. These results contrasted with what was reported until August 18, 2020, where there is a case fatality rate for hospitalized COVID-19 patients; 31.0% for the Secretary of Health in Mexico [22].
The COVID-19 pandemic highlighted the need for new and accurate diagnostic tools for RDs, but the pandemic has overwhelmed health systems globally, making it difficult to control the spread of the disease [23]. In Mexico, due to our particular public health and institutional structure, our situation is even more complicated.
These differences may be due to two types of factors:
1. - Patients are treated in different health sectors; IMSS, ISSSTE, SSA, and Private. 2. - Hospital factors specific to each sector. The COVID-19 pandemic places Mexico at particular risk since it should be noted that Mexico is among the first 10 countries with patients suffering from DM, some authors suggest that this disease affects about 9.2% of the population.
To the Archives and Statistics Department of the Zacatecas General Hospital “Luz González Cosío” of the Zacatecas Health Services, and to the staff of the Respiratory Disease Surveillance System (SISVER).
Note: In the abbreviations sometimes the acronyms will appear, not in the English order since they are taken from the Spanish.
- Mohan SV, Hemalatha M, Kopperi H, Ranjith I, Kumar AK. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem Eng J. 2021 Feb 1;405:126893. doi: 10.1016/j.cej.2020.126893. Epub 2020 Sep 4. PMID: 32901196; PMCID: PMC7471803.
- World health organization. Who disease outbreak news: novel coronavirus – republic of Korea (ex-china). January 21, 2020. Https://www.who.int/csr/don/21-january-2020-novel-coronavirus-republic-of-korea-ex-china/en/.
- https://coronavirus.jhu.edu/map.html 2020.
- Remes-Troche JM, Velarde-Ruiz Velasco JA. The Liver and COVID-19 in Mexico. Clin Liver Dis (Hoboken). 2021 Aug 27;19(2):49-52. doi: 10.1002/cld.1153. PMID: 34900238; PMCID: PMC8653117.
- https://coronavirus.jhu.edu/map.html. 2021.
- Rodriguez-Morales AJ, Gallego V, Escalera-Antezana JP, Méndez CA, Zambrano LI, Franco-Paredes C, Suárez JA, Rodriguez-Enciso HD, Balbin-Ramon GJ, Savio-Larriera E, Risquez A, Cimerman S. COVID-19 in Latin America: The implications of the first confirmed case in Brazil. Travel Med Infect Dis. 2020 May-Jun;35:101613. doi: 10.1016/j.tmaid.2020.101613. Epub 2020 Feb 29. PMID: 32126292; PMCID: PMC7129040.
- https://coronavirus.gob.mx/wp-content/uploads/2020/07/Lineamientos-Reconversion-Hospitalaria_05042020_2.pdf
- Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, Tkachuk VA, Markov AG, Lehnert H, de Angelis MH, Rietzsch H, Rodionov RN, Khunti K, Hopkins D, Birkenfeld AL, Boehm B, Holt RIG, Skyler JS, DeVries JH, Renard E, Eckel RH, Alberti KGMM, Geloneze B, Chan JC, Mbanya JC, Onyegbutulem HC, Ramachandran A, Basit A, Hassanein M, Bewick G, Spinas GA, Beuschlein F, Landgraf R, Rubino F, Mingrone G, Bornstein SR. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021 Nov;9(11):786-798. doi: 10.1016/S2213-8587(21)00244-8. Epub 2021 Oct 4. PMID: 34619105; PMCID: PMC8489878.
- Velarde-Ruiz Velasco JA, García-Jiménez ES, Remes-Troche JM. Hepatic manifestations and impact of COVID-19 on the cirrhotic patient. Rev Gastroenterol Mex (Engl Ed). 2020 Jul-Sep;85(3):303-311. English, Spanish. doi: 10.1016/j.rgmx.2020.05.002. Epub 2020 May 27. PMID: 32553772; PMCID: PMC7250745.
- Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020 Jun 22;62:e43. doi: 10.1590/S1678-9946202062043. PMID: 32578683; PMCID: PMC7310609.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Características clínicas de 138 pacientes hospitalizados con neumonía infectada por el nuevo coronavirus de 2019 en Wuhan, China. JAMA. 2020; 323: 1061–1069.
- Caussy C, Wallet F, Laville M, Disse E. Obesity is Associated with Severe Forms of COVID-19. Obesity (Silver Spring). 2020 Jul;28(7):1175. doi: 10.1002/oby.22842. Epub 2020 May 21. Erratum in: Obesity (Silver Spring). 2020 Oct;28(10):1993. PMID: 32314861; PMCID: PMC7264509.
- Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020 Jul;16(7):341-342. doi: 10.1038/s41574-020-0364-6. PMID: 32327737; PMCID: PMC7187148.
- Ren H, Guo X, Palazón-Bru A, Yang P, Huo N, Wang R, Sun Y, Hu Q, Yang H, Xu G. Regional Differences in Epidemiological and Clinical Characteristics, Treatment, and Clinical Outcomes of COVID-19 in Wuhan and Remote Areas of Hubei Province. Front Med (Lausanne). 2021 Jul 15;8:667623. doi: 10.3389/fmed.2021.667623. PMID: 34336881; PMCID: PMC8319467.
- Rodríguez Nancy P. Distribution of the population vulnerable to COVID-19 in Havana, Cuba. Revista Cubana de Higiene y Epidemiología. 2020; 57:371 https://creativecommons.org/licenses/by-nc/4.0/deed.es_ES
- Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31;36(7):e3319. doi: 10.1002/dmrr.3319. Epub ahead of print. PMID: 32233013; PMCID: PMC7228407.
- Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M; LICORN and the Lille COVID-19 and Obesity study group. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020 Jul;28(7):1195-1199. doi: 10.1002/oby.22831. Epub 2020 Jun 10. Erratum in: Obesity (Silver Spring). 2020 Oct;28(10):1994. PMID: 32271993; PMCID: PMC7262326.
- Pacheco-Pantoja E, Fernando A. Bravo F, Ángel E. ceballos-Cruz. COVID-19, diabetes, obesity and hypertension: 60 days of pandemic in Mexico. Rev Mex Endocrinol Metab Nutr. 2020; 7:68-79
- Calixto-Calderón B, María F. Vázquez-González, Martínez-Peláez R. Pre-existing comorbidity, the highest risk factor for poor prognosis of COVID19 among the Mexican population. DOI: https://doi.org/10.21640/ns.v13ie.2823
- Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021 Aug 21;21(1):855. doi: 10.1186/s12879-021-06536-3. PMID: 34418980; PMCID: PMC8380115.
- Slobdan P, Stuling T. Diabetes and covid-19. Springer Nature.2020 May; 132:356-361.
- https://datos.nexos.com.mx/la-letalidad-hospitalaria-por-covid-19-en-mexico-desigualdades-institucionales/
- Emara HM, Shoaib MR, El-Shafai W, Elwekeil M, Hemdan EE, Fouda MM, Taha TE, El-Fishawy AS, El-Rabaie EM, El-Samie FEA. Simultaneous Super-Resolution and Classification of Lung Disease Scans. Diagnostics (Basel). 2023 Apr 2;13(7):1319. doi: 10.3390/diagnostics13071319. PMID: 37046537; PMCID: PMC10093568.